For the SEB magazine I reported on the Biomechanics session at the SEB Annual Main Meeting in Valencia. This report covers self-sowing seeds, stag beetles,frog legs, tree frogs, sucking plants and plant-made plasters.
Seeds that sow themselves.
To distribute their seed, plants rely on animals, wind, water, fie or, in fact, biomechanics; from pine trees to cereals, many species possess mechanical mechanisms to actively scatter their seeds, that is, to self-disperse. A great example is the Geranium genus, which catapults its seeds away when the seed-awns bend, coil or twist. ‘These movements are hygroscopic’, explains Rivka Elbaum. ‘That is to say, seeds move in a certain fashion depending on how wet or dry they are.’ Rivka studies the structure and composition of cell walls that underlie these movements at the Hebrew University of Jerusalem in Israel. The cell wall’s cellulose fibrils are embedded in a matrix that absorbs water molecules. As the air gets wetter or drier, the cell wall takes up or loses water, causing it to swell or shrink, respectively. Depending on how the cellulose fibrils are oriented, swelling and shrinking cause different movements. ‘The Geranium awns possess cellulose fibrils that are wound around each cell in a helical fashion, like a telephone wire around a pencil’, explains Rivka. The angle of the cellulose helix in relation to the cell’s circumference determines how the awn moves during drying. At a constant angle, the cell wall contraction during drying causes a twisting movement. If the cellulose fibril angle varies the cells will coil. This coiling motion is found in the awns of the Geranium, Erodium gruinum, which propels the seed across the ground to a spot where it can germinate. ‘But that’s not all’, says Rivka. ‘As the air humidity changes with the day-night rhythm, the coiled seeds also expand and re-coil rhythmically.’ This causes a creeping movement as well as a drilling movement into the soil. This means, not only do these hygroscopic movements allow the seed to disperse, it literally sows itself as well.
Stag beetles use their muscle.
Stag beetles are so named thanks to the huge structure of their jaws, which resembles a stag’s antlers. These jaws are about the size of the beetle’s body and move like scissors to fight off competing males. ‘When stag beetles fight, they use their jaws to pinch their opponent, lift it up above its head and throw it down, so that the opponent often lands on its back’, says Jana Goyens from the University of Antwerp, Belgium. She explains, that, just like scissors, the two arms of the jaw behave as levers and a huge head-muscle controls their movement. ‘The force that this muscle can exert at a robust tip in the middle of one arm is around 7 Newtons’, says Jana, ‘which is a bite-force equivalent to balancing a 700g bag of flour on a toothpick, on your finger.’ At the fragile end tip of the jaws, the force is much smaller, around 2.5 Newton. Jana explains: ‘When you try to cut something hard with scissors it’s more difficult to cut with the scissor’s tip than closer to the hinge. This is the lever effect. At the tip of the scissors the force is smaller’. Jana discovered that the force exerted at the tip of the beetle’s jaw is smaller than would be expected for a mechanical lever of that size. She concluded that the beetle actively reduces the force exerted by its head muscle when trying to pinch an opponent with the tip of its jaws: ‘This is important because the tip of the jaw is much more fragile and the beetle cannot afford to break it whatsoever.’ So how does the beetle know when it is pinching something with its fragile end-tip, and to tune down its muscle force? Jana used electron microscopy and examined the jaw surface. She found structures resembling sensors that may sense the jaw material’s deformation while pinching. This sensing of deformation might be the signal for the beetle to modulate its force and so avoid injuring its vital weapon.
What do tree frogs and wet napkins have in common?
It is lunch break and Dr Jon Barnes takes a napkin, wets it with left-over beverage and throws it at the wall behind us, where it sticks. ‘This’, he exclaims, ‘is wet adhesion’. It is mainly caused by capillary forces and tree frogs use it to climb without falling off. Unlike the famous geckoes, which use dry adhesion, tree frogs extrude a watery fluid from their specialised toe pads and use wet adhesion to stick to surfaces. Toe pads feature hexagonal cells that are separated by deep channels. ‘Fluid spreads beneath the pad through these channels’ explains Jon, ‘to produce a uniform, but extremely thin layer of fluid beneath the pad, and an air-water interface around the perimeter of the pad. This generates a capillarity force’. Thomas Endlein, a postdoctoral researcher in Jon’s group, explains that there are two components to this effect: ‘Firstly, capillarity relies on the surface tension of a fluid, which also keeps a droplet of water rounded up, and secondly there is a pressure force, the force that causes water to rise up a glass capillary tube.’ But wet adhesion alone does not explain what keeps the frogs stuck. Niall Crawford, PhD student in the group explains: ‘If the surface itself is steep, then it is necessary to create friction.’ Jon’s group have found that the hexagonal cells of the frogs’ toe pads are covered with so-called ‘nanopillars’, which touch the surface and help to create friction. However, if the surface is very wet the air-water interface on which capillarity depends may be lost. Therefore, the group went to Borneo to examine torrent frogs that live by fast-flowing rivers and climb on surfaces that are wet. The team used a tilting platform to measure the angles at which frogs could no longer adhere to the platform’s surface. They found that both tree and torrent frogs adhered equally well when the platform was dry. As they flooded it with water, however, torrent frogs still clung to the tilted surface, when tree frogs had already fallen off. ‘We visualised the frogs’ areas of contact with the platform’, explains Jon, ‘and we saw that torrent frogs were able to use parts of their thighs and belly to adhere to the wet platform, whereas tree frogs did not.’ The torrent frog’s better adhesion was due to their increased surface area used for adhesion. Additionally, torrent frogs’ toe pads are developed to better get rid of excess fluid and so are better adapted to maintain close contact under wet conditions.
Frog legs – a recipe for success?
‘It might have happened to you that you lifted something that you expected to be heavier or lighter than it actually was, like a bucket of sand that turned out to be a bucket of ash’, says Christopher Richards. ‘If so, you will have experienced the interaction between your muscles and the load on them.’ Chris is a Research Fellow at the Royal Veterinary College at the University of London and studies how muscle force and speed is controlled by load, in other words, by the task that the muscle has to perform. He explains: ‘If I move my arm through the air the load on it is very small. But if I push it against a glass window, the window ‘pushes back’ with the same force and that constitutes the load on my arm. If I swim through water, my hand experiences the fluid-dynamic load of the water pushing against it, and if I artificially increase my hand size, say by attaching a paddle to it, I increase this load. This increase in load, in turn, feeds back to control my muscle force and speed.’ Of course, our nervous system is responsible for initiating movement, to ‘turn on’ the muscle and to prepare it for anticipated movement. The control of the muscle by the load, or by varying load, however, is much faster than can be realised through neurotransmission and Chris wants to find out how this control is actually achieved. To do so, he and his team have built a robotic frog leg with three joints and a frog leg’s fin, the size and shape of which can be varied. Chris and his colleagues used an isolated frog leg muscle, which is stimulated by an ‘artificial brain’. From the feed-back mechanisms built into the set-up they can deduce how different shapes and sizes of the frog leg’s fi modulate muscle force and speed. Says Chris, ‘The work we are doing might reveal some general design rules of how a limb needs to be constructed in order to carry out a specific range of movements. For example, an arm needs to be built to be able to hit a baseball as well as to play the piano. Our work might help reveal such basic design principles that may be useful to prosthetics.
Do it yourself sealing - Plants need no plasters.
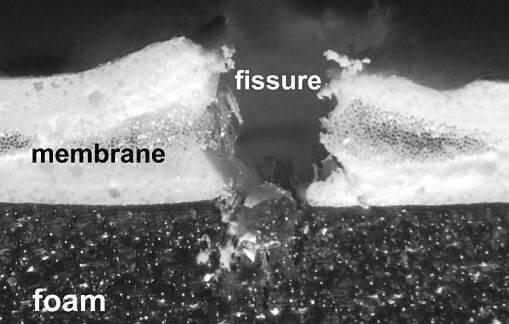
As sessile organisms, plants cannot flee their predators and are exposed to injury by, for example, insects or birds. Succulent plants that store a lot of water in their fleshy leaf tissue are especially at risk of dehydration if injured. Olga Speck of the Plant Biomechanics Group at the University of Freiburg studies how the Iceplant, Delosperma cooperi, achieves very fast self-sealing when pierced by a bird’s beak. ‘The key’, says Olga ‘lies in the anatomy of their near-cylindrical, fleshy leaves. You can see from a cross-section of the leaf, that it is composed of five shells, five layers of tissues with alternating mechanical properties.‘ At its core there is a stiff vascular bundle under pre-tension. It is surrounded by a layer of turgid tissue that stores a lot of water and so is under compression. The third layer of tissue consists of a net of vascular bundles, and is under mechanical tension. A fourth layer of turgid, water-storing tissue under mechanical pressure follows and finally an outer epidermis under tension. So tissues under pressure alternate with tissues under tension. In an intact leaf, both forces of pressure and tension are in equilibrium and constitute a mechanical stability. If this equilibrium is disturbed through injury the elastic energy stored is released and drives a movement that seals the wound. ‘It is a bit like a blown-up balloon’, says Olga. ‘The inside is under pressure and the balloon’s membrane is under tension. If you pierce it, the elastic energy stored in the antagonism of forces is released and drives a movement, namely a deflation. ‘ The movement of the Delosperma leaf depends on the depth and direction of the injury. In response to a lateral cut, for example, the leaf bends like an arm around an elbow and so closes the cut. ‘This movement is complete within about 60 minutes, which is quite fast, and is necessary to stop dehydration and prevent invasion of the wound by parasites or fungi’, explains Olga. ‘Only then can the process of wound healing begin.’
This plant sucks.
Carnivorous plants have fascinated Simon Poppinga since childhood, when his favourite cartoon character was captured in a Venus Flytrap. ‘Many carnivorous plants have to out-smart their often very fast prey by using what I call ‘cheap tricks’’, he says. ‘That is to say, it’s is a bit like maintaining a flexed bow and arrow, where elastic energy can be stored and released on demand, causing a movement.’ This is what many carnivorous plants do, amongst them the bladderworts (Utricularia spp.), which Simon studies at the University of Freiburg in Germany. These aquatic plants possess tiny bladders that are able to trap small prey and digest it. To capture prey, the bladders create an under-pressure inside and when this pressure is released by touch, a trap door opens and prey is sucked inside. ‘You can really say that this plant sucks’, Simon jokes, and explains: ‘It is a bit like drawing out the air from a plastic bottle with your lips. If you do that the walls of the bottle bend inwards, thereby storing elastic energy. As you remove the bottle from your lips, the walls regain their original shape, the energy is released and air is sucked into the bottle. This is what is happening in the bladderwort, but under water.’ This underlying principle has been known since Darwin’s times, but Simon and his colleagues have now resolved the mechanism of trap door opening using high-speed cameras. ‘It is a mechanism akin to that of the Venus Fly-trap, where the leaf structure’s shape changes from convex to concave’, says Simon. ‘Door opening relies on a flip-flip mechanism in which the trap door changes its shape.’ When the researchers stimulated the hair at the door opening, the door bulged towards the bladder-inside at a small region close to the trigger hair. This inversion of the door curvature then spread progressively along the whole trap door until the door flicked inside to open the trap. ‘This process happens within only half a millisecond’, says Simon, ‘and closing the door by regaining its original shape takes only 0.5 to 300 ms.’ These velocities may not sound like ‘cheap tricks’ but they certainly ‘out-smart’ the prey whizzing around the bladderworts’ pond.
Self-sealing foam in action. The fissure in the membrane is visible while the foam has closed its fissure and so seals the membrane. Photo: Plant Biomechanics Group Freiburg